Unique biophysical properties of the TRPV1 pore
back
TRPV1 has originally been cloned on
the basis of its ability to confer capsaicin (the spicy component of
chilli pepper) sensitivity when heterologously expressed in mamallian
cell lines (Caterina et al. 1997 Nature). By testing for additional
sensory qualities that are mediated by TRPV1, Caterina et al. found
that TRPV1 also responds to high temperatures and acidic extracellular
media. In addition other studies have demonstrated that TRPV1 is
further modulated by a number of receptor-mediated signalling pathways
including the activation of PKC, PKA or PLA2/LOX
pathways. Moreover, TRPV1 is released from a functional block by
PLC-mediated PIP2 hydrolysis. The mechanism of
TRPV1 potentiation by extracellular protons has been worked out and the
protonatable amino acids that are flanking the pore mouth and initiate
the proton-induced TRPV1 potentiation have been clearely defined.
TRPV1 is a poorly selective cation channel that is permeable to Na+,
K+, Ca2+ and Mg2+.
Despite the well-defined potentiation by extracellular protons, effects
of TRPV1 activation on the intracellular pH have not been studied.
Since other Ca2+-permeable cation channels have
been demonstrated to mediate at least a Ca2+-entry-mediated
intracellular acidification, we studied whether TRPV1 activation
affects the intracellular pH in a heterologous expression system as
well as in freshly isolated rat nociceptive dorsal root ganglion
neurons.
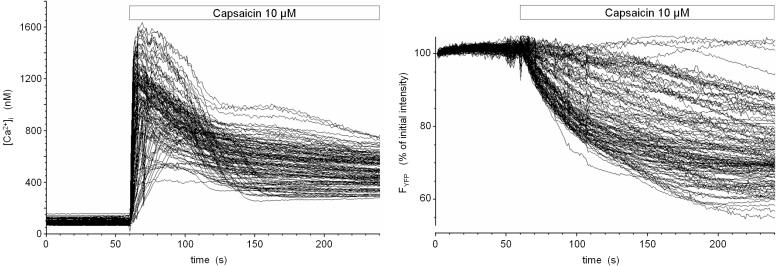
Fig. 1: Activation of TRPV1 in a TRPV1- and eYFP-expressing stably transfected cell line
The cDNA of rat TRPV1 was amplified and cloned into the mammalian expression vector pcDNA3. HEK293 cells were stably cotransfected with expression plasmids encoding TRPV1 and enhanced yellow fluorescent protein (eYFP). Upon fluorescence-assisted selection of single cell clones, cells were loaded with the low-affinity Ca2+ -indicator dye fura-4F and imaged ar 340 nm, 358 nm, 380 nm and 480 nm excitation in a digital video imaging system. Fluorescence intensities over regions of interest defined over single cells were converted into calibrated [Ca2+]i values (in nM) and are shown as time course during stimulation with 10 µM capsaicin (left panel). Coincident with the onset of Ca2+ influx, the fluorescence intensity of eYFP continously dropped (right panel). At that time, the pH- and chloride-sensitivity of yellow GFP variants has not yet been described, but the slower kinetics of eYFP fluorescence loss compared to the rapid increase and early peak signal of [Ca2+]i pointed to a calcium-dependent indirect process with intracellular acidification being a good candidate.
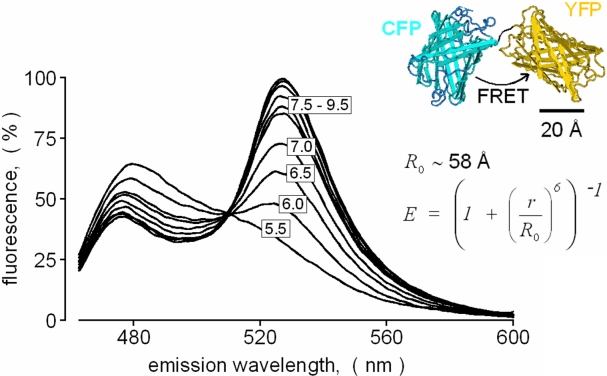
Fig. 2: Construction of a genetically encoded FRET-based pH sensor protein for calibrated pH imaging
The open reading frames of the
relatively pH-insensitive enhanced cyan fluorescent protein (eCFP-DStop) and
of eYFP were fused to obtain a mammalian expression vector encoding an
intramolecularly linked CFP-YFP-tandem protein. Upon stable expression
of the CFP-YFP-tandem in HEK293 cells, the fluorescent protein was
extracted, and spectral properties were analyzed in a fluorescence
spectrophotometer (Perkin Elmer LS-50B). Shown are emission spectra of
the tandem protein titrated to various pH values in a broad range
buffer. Excitation wavelength was 410 nm.
The tandem protein displays a huge pH-dependent spectral shift which is
suitable for pH calibration in imagang systems independently of the
local dye concentration. At basic pH, the eYFP-part of the tandem
protein is fluorescent, serves as a FRET acceptor (FRET efficiency
about 52% at pH 8.0) and emits at its typical emission maximum of 535
nm. Upon titration to lower pH values, the eYFP-part protonates
resulting in a loss of fluorescence and absorption at 420-520 nm.
Hence, the intramolecularly linked eCFP-part of the protein is
unquenched and emits more strongly at its own emission band (about
460-500 nm). Since the FRET efficiency scales directly with the
acceptor absorbance, the intracellular pH can be calibrated on the
basis of determined FRET efficiencies and the known pKa
of the eYFP-part of the tandem protein.
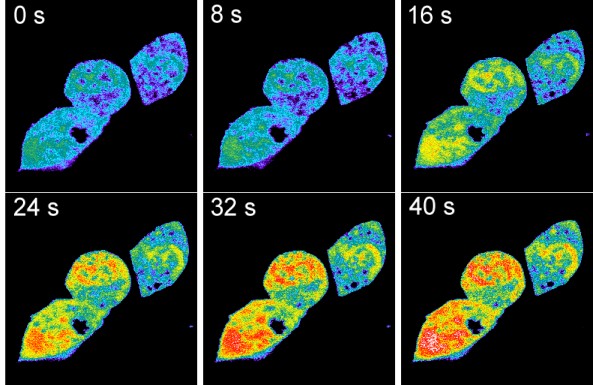
Fig. 3: TRPV1-mediated acidification in HEK293 cells imaged with the pH-sensing CFP-YFP-tandem protein.
HEK293 cells coexpressing TRPV1 along with the genetically encoded pH sensor were imaged by a spectrally resolving confocal microscope (Zeiss LSM-META). Capsaicin was added 20 s after the beginning of the image acquisition. Shown is a pseudocolored set of images taken before and after the capsaicin (10 µM)-induced intracellular acidification. The extracellular medium was a Ca2+- and Na+-free MES-buffered isotonic KCl solution adjusted to pHext 5.5. In this solution, a gradient for the permeation of mono- and divalent metal cations is lacking and protons are the only positive charge carriers that experience a driving force to enter the cell via the activated TRPV1 pore. The same effect was evident in Na+ or in Na+/Ca2+-containing solutions. To our knowledge, these were the first data providing evidence for a direct proton permeation through a classical water-filled ion channel pore in the presence of physiological concentrations of metal cations.
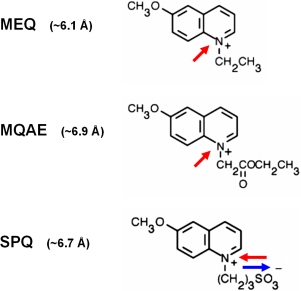
Fig. 4: Heterocyclic organic cations can permeate through the activated TRPV1 pore
The membrane-impermeable organic
fluorescent Methoxyquinolinium-based Cl--sensors
MEQ and MQAE were applied as optical probes for large cation permeation
through TRPV1. In the extracellular medium containing ~140 mVal Cl-,
the dyes are quenched. Capsaicin-stimulation led to an intracellular
loading of the dyes selectively into TRPV1-expressing cells. Due to the
lower intracellular Cl- concentration, the dyes
that are entring the cell are unquenched and can be easily detected by
fluorescence imaging. In contrast to the positively charged compounds
MEQ and MQAE, SPQ is an internal salt of and was, despite similar
molecular size than MQAE unable to permeate through TRPV1.
Besides the optical probes, electrophysiological data demonstrated
permeability for TEA+, bis-TRIS+,
bis-TRIS-propane+, histidine+
and NMDG+ thereby supporting the conclusion that
the activated TRPV1 pore has a diameter in the range of 5-7 nm.
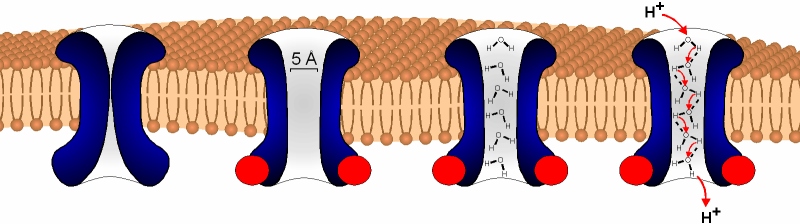
Fig. 5: Schematic illustration of the possible mechanism of proton permeation through TRPV1
Electrophysiological recordings demonstrated a 102- to 103-fold selectivity of proton permeation through TRPV1 compared to monovalent metal cations. Since TRPV1 poorly selects between classical metal cations, a distinct permeation mechanisms for protons is likely. Since the open pore-blocking agent ruthenium red blocked proton permeation through TRPV1, protons or hydrogen cations appeared to permeate through the pore. By probing the pore diameter, we found that bulky organic cations can pass through the activated TRPV1. Hence, the pore diameter (>5 µm) may be wide enough to become occupied by a continuous column of water molecules. In this case, proton would overcome diffusional limitations by "hopping" along the water molecules by hydrogenium cation formation and reorientation of the hydrogen-bonded network.
back
Original article:
Hellwig N, Plant TD, Janson W, Schäfer M,
Schultz G, Schaefer M (2004)
TRPV1
acts as proton channel to induce acidification in nociceptive neurons.
J. Biol. Chem. 279:34553-34561.