Homo- and heteromeric assembly of TRP channel subunits
back
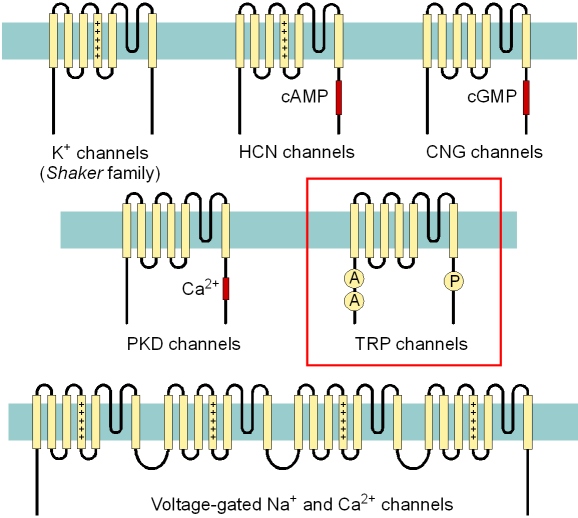
Fig. 1: Transmembrane topology of TRP channels and of distantly related cation channel families
TRP channels belong to the large
superfamily of cation channels with 6 transmembrane-spanning segments
S1-S6 forming a transmembrane domain with a pore loop inserted between
S5 and S6. In the pore-forming subunits of voltage-gated Na+
(NaV) and voltage-gated Ca2+
(CaV) channels, four transmembrane domain
repeats (with 6 transmembrane-spanning segments each) are
intramolecularly linked by three cytosolic loops. In analogy to first
structural data on the bacterial cation channels (Kcsa and KIR-Bac1.1),
the transmembrane domains presumably assemble in a ring-like structure
with four-fold symmetry and thereby contribute four pore loops as well
as their respective S6 and S5 segments that together align the singular
central pore with an outer gate, a selectivity filter, a pore vestibule
and, in some cases another inner gate.
In contrast to the NaV and voltage-gated Ca2+
channels, the intramolecular link between the transmembrane domains is
absent in voltage-gated K+ channels, in cyclic
nucleotide-gated (CNG) channels, in hyperpolarization-activated and
cyclic nucleotide-regulated (HCN) channels, in the polycystic kidney
disease (PKD) gene products and in TRP channels. These channel
families, thus, require four individual subunits in order to form the
pore.
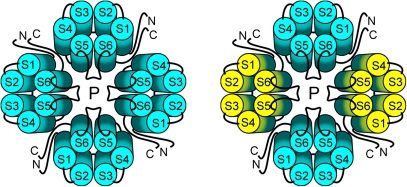
Fig. 2: The quartenary structure of TRP channels allows homo- or heteromeric configurations
Like previously demonstrated for Shaker-type K+ channels or CNG channels, the four-fold symmetry of TRP channel subunit assembly offers the potential of forming homo- (left) or heteromeric (right) channel complexes. Since each of the four subunits contributes to the central pore, regulatory and biophysical properties might be altered in heteromeric channel complexes compared to their respective homooligomers. First evidence of TRP channel heteromers was obtained for the eye-specific TRP/TRPL channel complexes in Drosophila melanogaster. The selectivity and promiscuity of mammalian TRP channel assembly was, however, largely elusive.
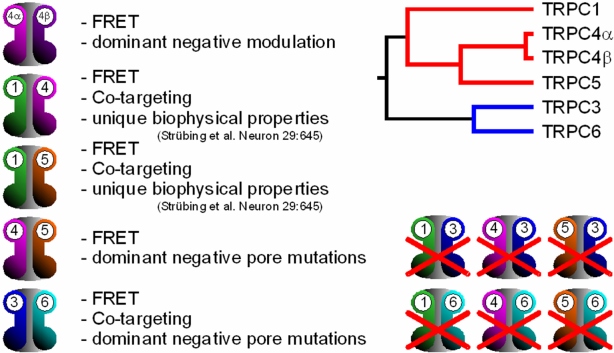
Fig. 3: Heteromeric TRPC channel complexes
To study the interaction between the TRPC4a and TRPC4b splice variants, we introduced fluorescence resonance energy transfer (FRET) to the assessment of the channel subunit assembly in living cells (Schaefer et al. J. Biol. Chem. 2002). The seven TRPC channel subunits TRPC1-TRPC7 constitute the first TRP channel subfamily in which we tested the potential of subunit heteromerization in a systematic manner. Imaging approaches, biophysical techniques, coimmunoprecipitation and functional data applying dominant negative mutants were applied to test the subunit association in living cells. The results demonstrate that only the closely related TRPC subunits 3, 6 and 7 or the other subgroup consisting of TRPC1, -4 and -5 were able to form heteromeric complexes. No association was evident across these phylogenetically more closely related subgroups. In line with our data, David Claphams lab (Strübing et al. Neuron 2001) has previously described TRPC1/TRPC5 heteromers that exhibit unique biophysical properties concerning the I/V relationship and single channel current amplitudes.
In Amiri et al. 2003, we applied a
competition-based approach for probing the specificity of FRET signals
in living cells. In homo- or heterooligomeric complexes, the FRET
efficiency does not only scale with the distance and the orientation
between fluorochrome-labeled interaction partners, but also with the
probability that the donor fluorochrome-fused interaction partner is
assembed with an acceptor fluorochrome-bearing counterpart. In
other words, if an excess of acceptor-fused subunits is expressed, the
FRET efficiency will be highest. Thus, a drop of FRET efficiencies can
be obtained either by expressing a molar excess of donor-labeled
interaction partners or by coexpressing unfused wild-type interaction
partners that occupy the protein-protein interaction interface
but
are not prone to oligomerisation due to a tendency of fluorescent
protein to aggregate in an unspecific manner.
By quantitatively assessing the FRET efficiencies and the relative
expression levels of cyan and yellow fluorescent TRPC6 channel subunits
in single cells, and by displacing this interaction by coexpression of
unfused TRPC6, we showed that the interaction is
specific. TRPC4,
a more distantly related member of the canonical TRP channel
family, failed to compete for the TRPC6 assembly, indicating a
strongly favoured homooligomeric assembly.
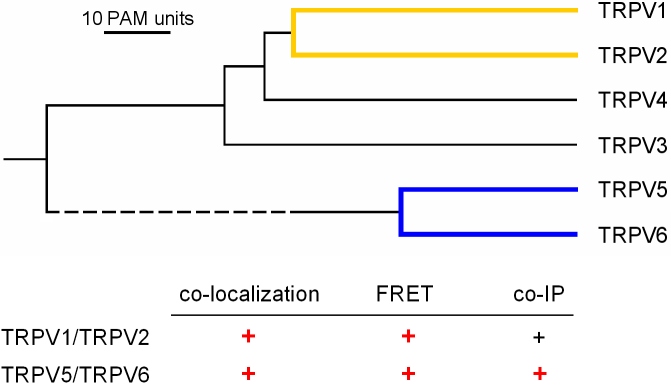
Fig. 5: Homo- and Heterotetramer formation between TRPV subunits
As a second TRP channel subfamily, we systematically probed the homo- and heterphilic oligomerization of the TRPV channel subunits TRPV1-TRPV6. Again, FRET, colocalisation analyses, and coimmunoprecipitation were applied. In contrast to the TRPC channel subfamily, heteromerisation between TRPV channel subunits was even more restricted. In the heterologous expression system, heteromeric TRPV channel complexes were only detectable between the most closely related TRPV subunits, namely TRPV5/TRPV6 and TRPV1/TRPV2. In all other cases, homomeric channel assembly was clearly preferred.
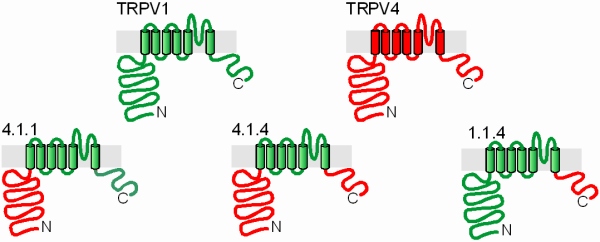
Fig. 6: Chimeric constructs of the non-interacting TRPV channel subunits TRPV1 and TRPV4 - a toolkit for probing the binding interfaces
The related, but physically not interacting TRPV subunits TRPV1 and TRPV4 were a good model for studying the distribution of binding modules on different parts of the subunits. To this end, conserved motivs in the end of the cytosolic N terminus and in the beginning of the intracellular C terminus were selected to fuse various combinations of the TRPV1 and TRPV4 termini to the respective transmembrane domain. Colocalization and FRET studies allowed the investigation of multimerization properties of these constructs independent of the channel function.
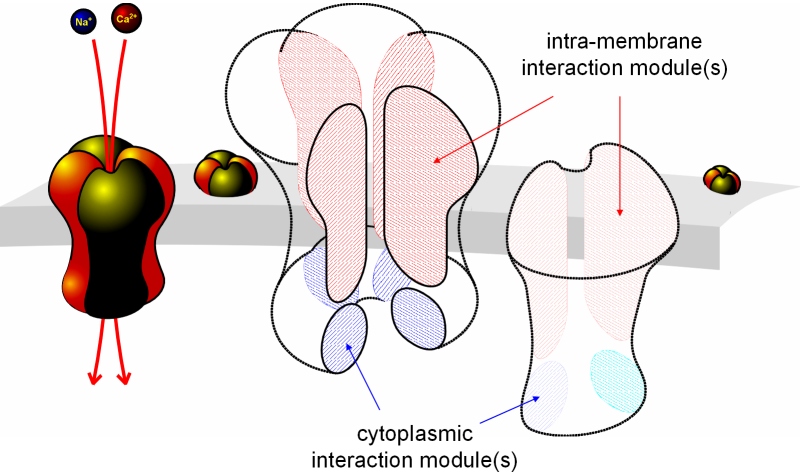
Fig. 7: TRPV channel assembly involves at least two interaction modules
Depending on the TRPV channel subtype, we found that either the termini or the transmembrane domain were sufficient to confer a binding affinity between chimeric TRPV channel constructs leading to FRET efficiencies similar to those seen in the respective native TRPV channel complex. In TRPV1, the transmembrane domain was sufficient to confer the multimerization to a TRPV4.1.4 construct or any other TRPV chimera that contained the common TRPV1 transmembrane domain. By contrast, TRPV4 binds to chimera that share the TRPV4 termini, but differ in the transmembrane domain. We suppose that at least two interaction modules exist and co-operate in the binding affinity and maintain the close contact over large parts of the inter-subunit interface and thereby stabilize the closed and open pore conformations. In order to maintain stable conformations of the outer and/or inner gates and the selectivity filter, channel subunit interactions possibly require multiple contact sites. Future work will concentrate on following this concept.
back
Original articles of the group covering the TRP channel multimerization:
Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer
M (2005)
Homo- and
heteromeric assembly of
TRPV channel subunits. J. Cell
Sci. 118:917-928.
Amiri H, Schultz G, Schaefer M (2003)
FRET-based
analysis of TRPC subunit stoichiometry. Cell
Calcium 33:463-470.
Hofmann T, Schaefer M, Schultz G, Gudermann T. (2002)
Subunit
composition of mammalian transient receptor potential channels in
living cells. Proc. Natl. Acad. Sci. USA 99:7461-7466.
Schaefer M, Plant TD, Stresow N, Albrecht N, Schultz G
(2002)
Functional
differences between TRPC4 splice variants. J. Biol.
Chem. 277:3752-3759.