The goal of our research activities is to bridge the knowledge gap between isolated, nanoscale particles in the gas phase and condensed matter. To this end, we develop methods to characterize the structure, reactivity and dynamics of clusters, nanoparticles and fluid interfaces using state-of-the-art mass spectrometric and laser spectroscopic techniques.
Our Research Projects
Nanoparticle samples are typically characterized by both physical and chemical heterogeneity. This leads, for example, to reduced performance in technological applications. Performance optimization requires insight into the intrinsic properties of individual nanoparticles and how they contribute to the properties of the ensemble. However, most experimental single nanoparticle methods study deposited particles whose properties are perturbed by either the carrier or neighboring particles. Characterizing only the intrinsic nanoparticle properties requires their isolation in the gas phase.
Research topics
- Single nanoparticle mass spectrometry
- UV/Vis- and IR-spectroscopy
- Determination of adsorption energies
- Elucidation of size-dependent effects in the size range from 5 to 100 nm
Publications
- B. Hoffmann, S. Leippe, K.R. Asmis
Probing the Electronic Absorption Spectrum of Single Gold Nanoparticles in the Gas Phase
Article in Mol. Phys. accepted (2023)
10.1080/00268976.2023.2210454 - B. Hoffmann, T.K. Esser, B. Abel, K.R. Asmis
Electronic Action Spectroscopy on Single Nanoparticles in the Gas Phase
Letter in J. Phys. Chem. Lett. 11, 6051−6 (2020)
10.1021/ACS.JPCLETT.0C01945 - T.K. Esser, B. Hoffmann, S. Anderson, K.R. Asmis
A Cryogenic Single Nanoparticle Action Spectrometer
Featured Article in Rev. Sci. Instrum. 90, 125110 pp. 1-10 (2019)
10.1063/1.5128203
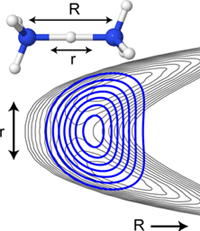
The properties of water and the particles dissolved in it are largely determined by the locally formed hydrogen bond networks. A detailed understanding of these interactions is of interdisciplinary interest. For example, hydrogen bonds play a central role in the description of proton transfer reactions (enzymatic catalysis), the dissolution behaviour of inorganic salts (solvation), the tertiary structure of biomolecules (peptide and protein folding) or the formation and properties of aerosols (nucleation) in the atmosphere, which is still far from being well understood at the molecular level.
By means of vibrational spectroscopic investigations on gas phase clusters of the type X(H2O)n, structural motifs as a function of the degree of hydration n (n = 0...~50) and of the temperature (T = 6 - 350 K) are investigated under well-defined conditions. By stepwise addition of single water molecules to individual ions (e.g. X = H+, e‒, SO42-, HSO4‒, NO3‒, HCO3‒, H2PO4‒, ‒OOC-(CH2)m-COO‒), ion pairs (e.g. X = MgNO3+, NaSO4‒, LiCl2‒, LiClI‒, LiI2‒) and deprotonated acid clusters (e.g. X = HSO4‒ (H2SO4)1-3, NO3‒(HNO3)1-3), the structural transition from an isolated to an almost completely hydrated particle can be followed spectroscopically. The strength of the formed X-H2O hydrogen bonds determines whether the hydration shell completely or only partially envelopes an ion, from when and in which form ion pairs are dissolved and which structural changes accompany progressive solvation. The IR spectra of simultaneously present constitutional isomers can be measured isomer-specifically over almost the entire IR range (200-4000 cm-1) using IR2MS2 spectroscopy. By specific addition of molecules with different proton affinities, the hydrogen-bonded network can be distorted in a controlled manner, e.g. to monitor structural changes spectroscopically.
Current Research Projects
-
Role of nuclear quantum effects in water adsorption and hydrogen bonded networks
-
Protonated water clusters / Grotthuß mechanism
-
Microhydrated ion pairs
-
Microhydrated ions with atmospheric chemical relevance
Current Publications
-
A. Chakraborty, T. Brumme, S. Schmahl, H. Weiske, C. Baldauf, K.R. Asmis
Impact of Anion Polarizability on Ion Pairing in Microhydrated Salt Clusters
Chem. Sci. 13, 13187-200 (2022)
10.1039/D2SC03431J -
A. Chakraborty, S. Schmahl, K.R. Asmis
Isomer-Specific Vibrational Spectroscopy of Microhydrated Lithium Dichloride Anions: Spectral Fingerprint of Solvent-Shared Ion Pairs
Communication (very important paper) in ChemPhysChem 22, 1036–41 (2021)
10.1002/CPHC.202100170 FRONT COVER - H. Knorke, H. Li, Z. Liu, K.R. Asmis
Vibrational Spectroscopy of the Hexahydrated Sulfate Dianion Revisited: Role of Isomers and Anharmonicities
Article in Phys. Chem. Chem. Phys. 21, 11651-9 (2019)
DOI: 10.1039/C9CP01802F - C.T. Wolke, J.A. Fournier, L.C. Dzugan, M.R. Fagiani, T.T. Odbadrakh, H. Knorke, K.D. Jordan, A.B. McCoy, K. R. Asmis, M.A. Johnson
Spectroscopic snapshots of the proton transfer mechanism in water
Science 354 1131-5 (2016)
DOI: 10.1126/science.aaf8425
German press release YouTube video Perspective
Review Articles
-
N. Heine and K.R. Asmis
Cryogenic Ion Trap Vibrational Spectroscopy of Hydrogen-Bonded Clusters Relevant to Atmospheric Chemistry
Int. Rev. Phys. Chem. 34 1-34 (2015). Corrigendium: 35 507 (2016).
DOI: 10.1080/0144235X.2014.979659
DOI: 10.1080/0144235X.2016.1203533 -
K.R. Asmis and D.M. Neumark
Vibrational Spectroscopy of Microhydrated Conjugate Base Anions
Accts. Chem. Res. 45 43-52 (2012).
DOI: 10.1021/ar2000748 .
Metal oxides play an increasingly important role as novel materials in a wide range of technical applications, e.g. as construction materials, as coatings, or in medical implants. The surface chemistry of these oxides is determined by their interaction with water and the ions dissolved in it. To optimize the lifetime (corrosion resistance) of such materials, a molecular understanding of the metal oxide/water interaction (oxide formation as well as oxide dissolution) is essential. Although there are already a large number of studies available on these interfaces, a fundamental understanding of this interaction at the molecular level is largely lacking.
This project aims to characterize the structure and ultrafast dynamics of model systems containing strong hydrogen bonds. Hydrogen bonding interactions are key to understanding the structure and properties of water, biomolecules, self-assembled nanostructures, and molecular crystals. However, there is still much confusion about their electronic nature, a combination of van der Waals, electrostatic, and covalent contributions leading to a variety of hydrogen bonds with bond strengths ranging from 2 to 40 kcal/mol (a 20-fold range!). In particular, our understanding of strong, low-barrier hydrogen bonds and their central role in enzyme catalysis, biomolecular recognition, proton transfer across biomembranes, and proton transport in aqueous media is still incomplete.
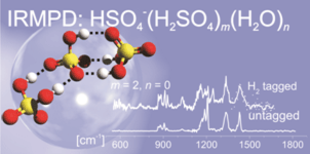
Inorganic acids (AH), their conjugate base anions (A‾), and water play critical roles in aerosol formation in the atmosphere. In the process of ion-induced nucleation, negative ions serve as more effective nucleation sites than positive ions. The most common anions in the troposphere and stratosphere include nitrate and bisulfate, as well as their microsolvated clusters. To understand the early steps of aerosol nucleation and gain insight into the microscopic structure of the bulk, we use infrared single and multiphoton dissociation spectroscopy (IRMPD) in combination with electronic structure calculations as a structural probe for size-selected (A‾)(AH)m(A'H)m'(H2O)n(H2)z clusters.
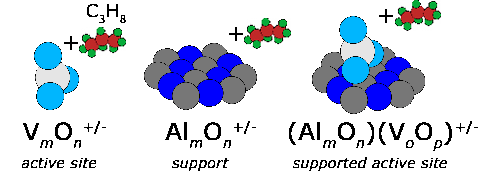
Transition metal oxides play a central role in the industrial use of heterogeneous catalysis. Interestingly, the underlying molecular reaction mechanisms are rarely known in detail. However, an understanding of these is necessary to contribute to the development of concepts for the preparation of catalysts with increased yield and selectivity. Within the framework of SFB 546 "Vanadium oxide aggregates" (1999-2011), we have built up a long-standing expertise in this respect, how spectroscopic studies of the structure, reactivity and dynamics on tailored metal oxide clusters in the gas phase can be used to investigate fundamental questions using this simple model system. This includes, for example, studies (a) on the correlation between local structure and reactivity, (b) on the identification of the spectroscopic fingerprint of active groups, (c) on the interaction between active groups and support materials, (d) on the calibration of quantum chemical computational methods, and (e) on the identification of reactive intermediates.
![[Translate to English:] Wirkungsspektroskopie an einzelnen Nanopartikeln. enlarge the image: Abbildung der Wechselwirkung eines Nanopartikel mit Laserstrahlen in einer Paulfalle.](/fileadmin/_processed_/0/7/csm_2020-01-22_artist_view_close_4_3_dd62d1f317.png)
![[Translate to English:] Wirkungsspektroskopie an einzelnen Nanopartikeln. enlarge the image: Abbildung der Wechselwirkung eines Nanopartikel mit Laserstrahlen in einer Paulfalle.](/fileadmin/Fakult%C3%A4t_Chemie/Institute/WOI/AK_Asmis/Bilder/2020-01-22_artist_view_close_4_3.png)