Professors Tobias Langenhan and Peter W. Hildebrand, scientists from the Faculty of Medicine at Leipzig University, and Professor Markus Sauer from the Julius Maximilian University of Würzburg, have researched adhesion GPCRs. These are a large but as yet poorly understood class of surface proteins that serve as cell sensors for chemical and mechanical stimuli. Adhesion GPCRs have a hidden, bound activator, or “tethered agonist”, that initiates signal transduction of the receptor. In the joint study, the three scientists found out how this agonist becomes visible for the receptor.
Adhesion GPCRs conceal the switch inside the GAIN domain, a ball-shaped portion of the receptor incorporated into the structure of the sensor protein. “We have shown that the crucial GAIN domain, which is present in all adhesion GPCRs, is enormously dynamic and flexible. This flexibility could have a decisive influence on the signalling by these receptors. It therefore seems plausible that in the future these could be modified by drugs in order to alleviate diseases which involve defective adhesion GPCR signals,” said Professor Langenhan from the Rudolf Schönheimer Institute of Biochemistry.
Adhesion GPCRs are medically and pharmacologically important molecules because their dysregulation plays a significant role in carcinogenesis. They also have a central function in the development of the brain and in immune processes. Adhesion GPCRs belong to the large family of G protein-coupled receptors (GPCRs). There are about 700 variants in humans, which are responsible for sensory impressions (e.g. sight, smell and taste), hormonal cycles, controlling the cardiovascular system and more. GPCRs translate stimuli that hit a cell from outside into an intracellular biochemical signal. It is estimated that almost half of all prescription drugs, such as those used to treat hypertension, Parkinson’s disease and allergies, act via GPCRs and their signals, the researchers say.
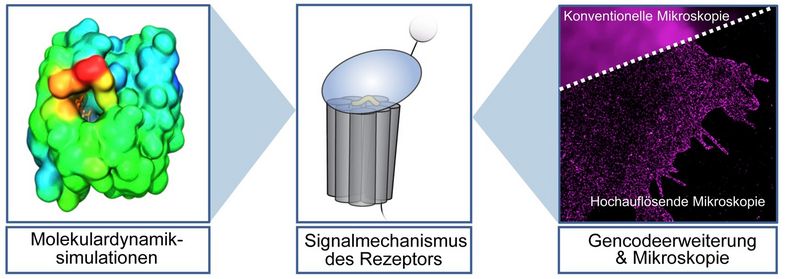
In an innovative approach, the scientists from Leipzig and Würzburg combined several methods to detect the unmasking of the adhesion GPCR switch. First, molecular dynamics simulations led by Professor Hildebrand at the Institute of Medical Physics and Biophysics served to calculate, using computer algorithms, how the GAIN domain of adhesion GPCRs behaves in space and time. “We managed to breathe life into the rigid structures of proteins by rendering them dynamic. This allowed us tp reveal functions of the protein that were not visible before,” said Hildebrand, who looked at the microscopic movements at an atomic scale of one ten-billionth of a metre on the computer.
In order to confirm the computer-based calculations, Professor Sauer from the University of Würzburg’s Biocenter and Professor Langenhan used various experimental methods. These included artificially expanding the genetic code of several tested adhesion GPCR genes and incorporating unnatural amino acids at some positions of the switch. Since the unnatural amino acids can only be labelled with a fluorescent dye when the switch is exposed from the GAIN domain, this method allowed the scientists to test its accessibility experimentally in complete adhesion GPCRs in cells. “These methods complemented each other very well to provide evidence that adhesion GPCRs can reveal their activator,” said Sauer, who imaged the receptor using dSTORM, a super-resolution fluorescence microscopy method.
“Unlike other GPCR representatives, so far there have been few ideas on how to change the signals of adhesion GPCRs. Our work has now identified a new target for this: the molecular movements of the GAIN domain,” said Langenhan. The three researchers plan to pursue this lead in new projects in the future.
Professor Markus Sauer from the University of Würzburg’s Biocenter is an internationally renowned expert in super-resolution microscopy. At the end of 2020, the European Research Council (ERC) awarded him and two professors from Göttingen and Massachusetts an ERC Synergy Grant of eleven million euros. The experts hope to use the money to improve medical imaging of functioning and pathologically altered nerve cells.
Professor Tobias Langenhan is head of the Chair of General Biochemistry at the Rudolf Schönheimer Institute at Leipzig University’s Faculty of Medicine. His work as a biochemist since 2016 has played a key role in shaping the local research profile. As spokesperson of the DFG-funded Research Unit (FOR) 2149 and a member of the new Collaborative Research Centre (CRC) 1423, which is receiving approximately 11.3 million euros in funding, his work currently includes investigating the structural dynamics and signalling behaviour of GPCRs.
Professor Peter Hildebrand has been Professor of Biophysical Computer Simulations at the Institute of Medical Physics and Biophysics at Leipzig University’s Faculty of Medicine since 2017 and, since 2018, a visiting professor at the Berlin Institute of Health (BIH), where he is investigating the influence of dynamics on the function of GPCRs together with Nobel Laureate in Chemistry Professor Kobilka of Stanford University. In his research, he develops methods to visualise the dynamics of proteins and investigates the pharmacology of G protein-coupled receptors, including in the Collaborative Research Centre (CRC) 1423.
Original title of the publication in Molecular Cell:
“Tethered agonist exposure in intact adhesion/class B2 GPCRs through intrinsic structural flexibility of the GAIN domain”, doi.org/10.1016/j.molcel.2020.12.042