About 40 per cent of all drugs act on G protein-coupled receptors (GPCRs), providing relief or even cures for a wide range of diseases. They include compounds used to treat Parkinson’s disease, pain, high blood pressure and allergies. GPCRs are easy for drugs to reach because they are located on the surface of cells. In addition, every cell in the body and its organs has very specific GPCR patterns, which means that the side effects of GPCR drugs can be well limited. “However, one large family within the more than 700-member GPCR class, the adhesion GPCRs (aGPCRs), is still uncharted pharmacological and pharmaceutical territory. These molecules are associated with a wide range of diseases, from cancer to psychiatric disorders,” explains Dr Nicole Scholz, group leader at the Chair of General Biochemistry at the Rudolf Schönheimer Institute and one of the two leaders of the new study.
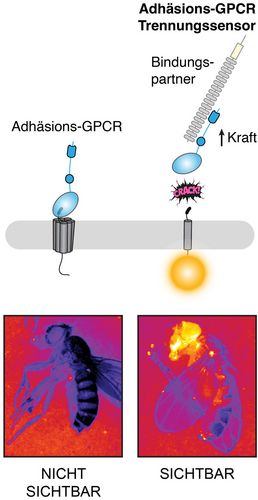
Adhesion GPCRs are a large class of surface proteins that recognise chemical and mechanical stimuli in the body. They have not yet been exploited for therapeutic drugs. aGPCRs have a two-component structure and a unique activation mechanism. The scientists from Leipzig have now presented a molecular sensor system that can be used in living organisms and in cell culture dishes to detect when and where an aGPCR breaks apart as a result of mechanical stimulation, thus separating the two components. This break can activate the receptors and thus plays a crucial role in the transmission of the biochemical signal. Dr Scholz and Professor Langenhan have filed a patent application for this technology in close collaboration with the Technology Transfer Office of Leipzig University. The aim is to provide legal protection for the new findings and to help bring the methods now available for aGPCR drug identification to the point of practical application.
Important biological process
“There is a notion that many aGPCRs are activated like hand grenades. The two parts of the aGPCR are like the safety pin and the explosive charge. When the safety pin is removed by mechanical stimuli and receptor binding molecules, the explosive charge is armed in the form of receptor activity. Through our work, we can now offer a method to render this mechanism visible. In particular, we have been able to show in which cells receptor separation occurs and under what conditions,” says Tobias Langenhan, Professor of General Biochemistry at the Rudolf Schönheimer Institute and co-leader of the study with Dr Scholz.
Dr Scholz adds: “We have succeeded in visualising an important biological process of a large receptor family in the living animal, the fruit fly. Future projects will include translating these findings to human aGPCRs. In the best case scenario, in the long term, we will be able to find compounds that modulate the activity of these receptors, and develop drugs that can treat the symptoms of adhesion GPCR-related diseases.”
It is precisely for this project that the inventors have now been recommended for funding by the German Federal Ministry of Education and Research, which will enable them to deepen their current findings and validate the process for commercial use. “Leipzig as a centre of research has been of great importance for the development of the NRS process. It brings together probably the largest number of research groups working on adhesion GPCRs in the world and provides a critical scientific environment,” says Professor Langenhan.
Original publication in Nature: Molecular sensing of mechano- and ligand-dependent adhesion GPCR dissociation. DOI: 10.1038/s41586-023-05802-5
Important contributions to this publication were made by DFG Research Unit FOR 2149, Elucidation of Adhesion-GPCR Signalling, which existed from 2015 to 2022. The current work was also supported by Collaborative Research Centre 1423 at Leipzig University, Structural Dynamics of GPCR Activation and Signaling.